In protein manufacturing, membrane proteins are well known to be challenging to produce in a functional form. SYNTHELIS’ patented cell-free technology allows customized expression and characterization of active proteins integrated in proteoliposomes. Membrane proteins are embedded directly into liposomes during the cell-free synthesis process allowing the proper folding of the protein. Following protein production, SYNTHELIS conducts quality controls to validate the target protein in terms of activity and structure.
Force spectroscopy is widely used in air, liquids, and different controlled environments. It may involve functionalized tips to study specific interactions of conjugated molecules. Molecular recognition measurements using AFM are based on the interaction between two molecules. One molecule is attached to the tip of the AFM whereas the second molecule is attached to the sample surface. Tip functionalization is the several chemical steps that lead to attaching the molecules to the tip of the AFM. The objective of this study wasto identify and characterize two transmembrane proteins by an approach of his-tag recognition by tris-Ni+-NTA modified tip (Figure 1). These studies show that single-molecule AFM has a great potential for studying the distribution of transmembrane proteins around liposomes. The following assays were performed in a collaboration between SYNTHELIS and BioMeca.
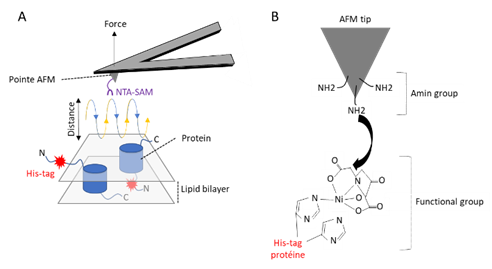
Figure 1: AFM mapping of NitriloTriacetic Acid - Self Assembled Monolayer (NTA-SAM) binding events using a chemically functionalized AFM tip. (A) Mapping adhesion forces on membrane proteins using FD-based AFM. When recording an AFM topography an approach (blue) and retraction (yellow) cycle between AFM tip and biological sample is performed for every pixel of the image. In each cycle, the cantilever deflection and the distance travelled by the AFM tip is monitored and transformed into an approach and retraction FD curve (B) Functionalization of AFM tip (tris-Ni+-NTA).
Material and Methods
Cell-free expression of proteoliposomes
SYNTHELIS was in charge of the production and the supply of the membrane proteins integrated into the lipid bilayer of the liposomes.
Thus, the twomembrane proteins retained for this study were expressed in a proteoliposome format using SYNTHELIS’cell-free expression system based on E.coli strain.Both proteins are 7-transmembrane domain (7TMD) receptors: one is a confidential target, the second one is a GPCR: C-X-C chemokine receptor type 4 (CXCR4).
Eachprotein hasa poly-His tag at the N-terminal end.
AFM tip functionalization
Gold tips (NPG-10, Bruker Nano AXS, Palaiseau, France) were covered with NTA-SAM after overnight incubation in a solution of NTA-SAM (Prochimia, Poland) 0.1 mM in ethanol. Then the tips were rinsed extensively with ethanol, dried with nitrogen and incubated for 1h in 40 mM NiSO4 in PBS solution and stored at 0-5 °C.
FD-based AFM
A multimode 8 AFM with a Nanoscope 5 controller (Bruker, Santa Barbara, California, USA) was operated in the ‘PeakForceTapping’ mode. The AFM was equipped with a 120 μmpiezoelectric scanner (J scanner). Rectangular cantilevers with nominal spring constants of ≈ 0.06-0.12 Nm-1 and a resonance frequency of ≈18 kHz in water were chosen. All AFM experiments were done in imaging buffer solution at room temperature (≈ 24 °C). Adhesion maps were obtained by oscillating the functionalized tip at 0.25 kHz, with an amplitude of 25 nm, and applying an imaging force of 100 pN. Topographs of 128x128 or 256x256 pixels were performed scanning 0.125 line per s. The retraction speed was 1500 nm/s and the contact time between tip and sample was 500 ms.
Data analysis
The force versus curves of each interaction recognition experiment were saved and exported as text files. NanoScope Analysis v1.9 and BiomecaAnalysis were usedto translate force versus time curves into FD curves showing specific adhesion events. The obtained force versus distance curves were then analyzed on the basis of the WLC (Worm LikeChain) model. This model is the most suitable and frequently used to describe the extension of polypeptides. The extension z of macromolecule is related to the retraction force Fadh via(eqn(1)) :
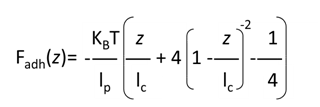
Wherethe persistence length lp is a direct measure of the chain stiffness, lc is the total contour length of the biomacromolecule and kb is the Boltzmann constant. The number of monomers in the polypeptide chains was then derived from following equation (eqn(2)) :
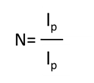
Results and Discussion
After having shown that the functionalized AFM tip can either detect the His-tag site of proteins, we want to investigate whether we could use the tip to detect specific adhesion.
To distinguish between the rupture events of tip without tris-NTA and liposomes surface we set-up a force filter. As measured above, the strength of tris-NTA and his-tag bond ranges from 100 to 250 pN, which is the characteristic force required to separate the bond formed between tris-NTA and his-tag. We thus designed the force filter to discard all rupture between 0 and 100 pN.
7 TMD receptor: confidential target
This receptor is similar to a G protein-coupled receptor (GPCR), but it is oppositely orientated to GPCRs in the cell membrane. Thus, the poly-His tag at the N-terminal end of the protein must be inside the proteoliposomes.
No adhesion force between the NTA tips and proteoliposomes was first detected. After a treatment of the samples with a triton detergent in order to solubilize theproteoliposomes and have access to the N-terminus of the proteins, 8 adhesions were detected.
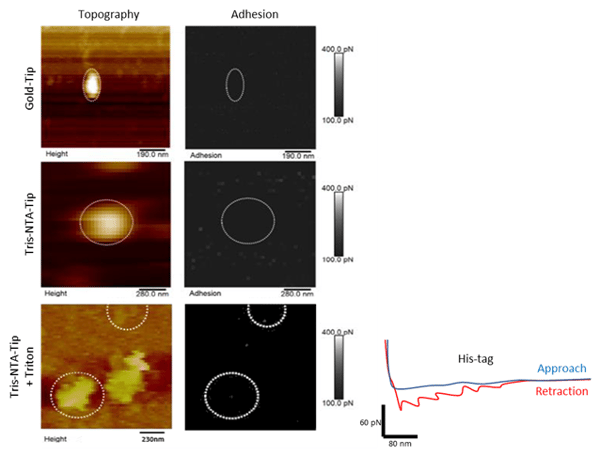
Figure 2: Mapping specific interaction forces between the tris-NTA-modified AFM tip and His-tag. (A, B and C) Topography map of liposomesabsorbed onto a freshly cleaved mica support. (A’, B’ and C’) Adhesion map of specific adhesion forces ranging between 100 and 400 pN.FD curve of each pixel detecting an adhesion event in the adhesionmap (grey circle). (D)Red-color FD curve detect specific adhesion event that are clearly separated from tip-support contact region.
GPCR: CXCR4 receptor
CXCR4 has an extracellular N-terminus end, seven transmembrane alpha helices connected by three extracellular and three intracellular loops, and a C-terminus end that is located in the cytoplasm.
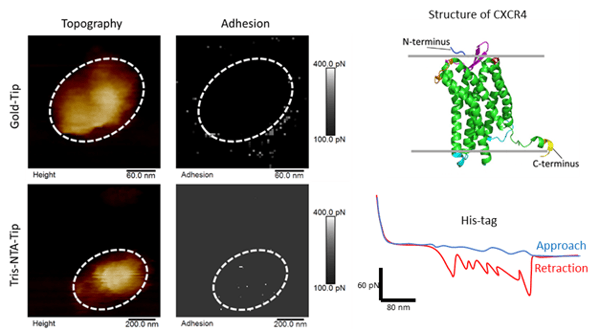
Figure 3: Mapping specific interaction forces between the tris-NTA-modified AFM tip and His-tag. (A and B) Topography map of liposomesabsorbed onto a freshly cleaved mica support. (A’ and B’) Adhesion map of specific adhesion forces ranging between 100 and 400 pN. (C) Structure of CXCR4. FD curve of each pixel detecting an adhesion event in the adhesionmap (grey circle). (D) Red-color FD curve detect specific adhesion event that are clearly separated from tip-support contact region.
Characterization of membrane proteins embedded in the lipid bilayer of liposomes is not easy to investigate. The proper orientation of the protein can be estimatedthrough a kinetic study of the enzymatic cleavage of the protein tag monitored by Western Blot analysis. The protein density is also usually estimated using Cryo-EM.
As shown in this application note, AFM approach provides, with one single instrument, information about the protein density, the protein orientation and the protein folding in the lipid bilayer of a complex material as liposomes. In addition, AFM technology does not require the labelling of the proteins which renders this approach quite straightforward to use as a Quality Control test. AFM might thus have a potential to become a standard method to control protein quality in the bioproduction processes.






