Synthelis' cell-free liposome-based expression system was applied in the development of an OprF vaccine against Pseudomonas aeruginosa. The complete OprF outer membrane protein of P. aeruginosa was used as the immunogen and was expressed in its native form in liposomes.
The resulting OprF proteoliposomes were fully characterized and their ability to induce an immunogenic response in mice was tested. The results showed the attractive features of the developed recombinant vaccine and its effectiveness in inducing a protective humoral response against P. aeruginosa, producing neutralizing antibodies that prevented or cured P. aeruginosa infections. The developed vaccine shows great promise as a candidate for human use.
Pseudomonas aeruginosa: major cause of nosocomial infections
Pseudomonas aeruginosa is a Gram-negative bacterium and a major responsible for nosocomial infections and pneumonia in hospital environment, frequently associated with the use of hospital devices such as catheters, central lines and ventilators [1]. P. aeruginosa also colonizes the respiratory tract of patients with obstructive lung diseases, resulting in chronic infections that can be lethal [2]. Furthermore, P. aeruginosa is known to present a high capacity to develop resistances to antibiotics [3]. Together, all these aspects lead to the urgent need for new antimicrobial strategies that allow to reduce the burden of P. aeruginosa infections and the associated morbidity and mortality. Considering the ability of this agent to develop or acquire new resistance mechanisms, the use of a vaccine to prevent infections constitutes a valuable and clever alternative approach to surpass the current problem of P. aeruginosa antibiotic resistance [4,5].
The outer membrane protein F (OprF): a good target for a vaccine development
The outer membrane proteins of Gram-negative bacteria are useful targets for the development of vaccines since most of them expose epitopes of the bacterial surface that can be recognized by the host immune system. In P. aeruginosa, the outer membrane protein F (OprF) seems particularly suitable for this purpose, as it is highly conserved and antigenically related among the serotypes of P. aeruginosa [6]. Moreover, it participates in diverse infection events such as biofilm formation, outer membrane vesicles biogenesis, binding and adhesion to host cells, involvement in quorum-sensing response and sensor of host immune activation through IFN-γ binding [7,8]. Structurally, OprF is a porin, presenting closed and open channel conformations. In the closed conformation, the N-terminal part spans eight times through the membrane, whereas the C-terminal part is located in the periplasm [9,10]. In its open conformation, the C-terminal part is located in the outer membrane, forming with the N-terminal part an integral membrane protein [10]. The closed channel conformation is the most represented (95%) and has a structural role in the bacterial cell wall.
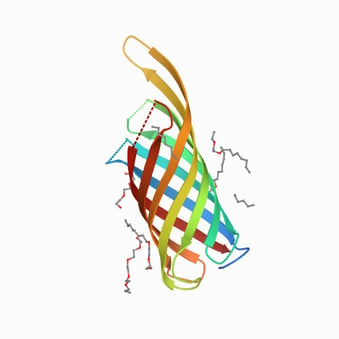
OprF protein structure
Cell-Free protein synthesis, a solution for OprF production
In spite of the favourable characteristics of OprF to be used as an immunogen in a vaccine, it also presents some problems, since it is difficult to develop a vaccine against a multispanning membrane protein, in its native conformation. Furthermore, it exhibits cellular toxicity on induction and gives low yields of correctly folded recombinant protein [11]. To surpass these limitations, a cell-free protein synthesis (CFPS) system can be used, in which the expression of membrane proteins is not impaired by their potential lethality [12] and can easily be adapted for large-scale protein production [13]. In the presence of liposomes or nanodiscs, cell-free systems allow the membrane protein to be translated and spontaneously inserted in the liposomal membrane in a one-step reaction [14]. In addition, CFPS systems enable the reconstitution of the complete membrane protein in its active form, presenting the native antigens [12,15].
The development and characterization of a OprF-based vaccine against P. aeruginosa was reported in 2021 [16] by using E. coli-based cell-free expression, in which the full-length OprF protein of P. aeruginosa was reconstituted in proteoliposomes, in the presence of synthetic liposomes. Fusion of the recombinant proteoliposomes into preformed tethered lipid bilayer membranes resulted in the incorporation of OprF in its native closed conformation and also, for a large proportion (36%), in its rare active open conformation, which resulted in the formation of mega-pores across the liposomal membrane.
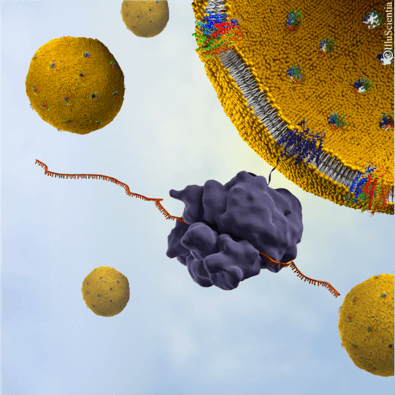
Illustration of liposome-based Synthelis expression system
Features of the OprF proteoliposomes
First, the authors optimized the lipid composition of the liposomes, which allowed to obtain protein yields from 0.5 to 1 mg/ml. The subsequent two-step purification process enabled to get highly pure (around 95% purity) OprF proteoliposomes. Most of the OprF proteins were shown to be embedded into the liposomal membrane in the correct orientation and in accordance with the two transmembrane topologies (closed and open channel conformers). Furthermore, the formation of large OprF pores in the liposomes was observed, with an average diameter of 9.5 ± 4 nm, which could be due to OprF oligomerization.
The OprF porin activity was assessed and it was shown that the porin was permeable to KCl. The influence of interferon-gamma (IFN-γ) was evaluated on the ion channel activity, since it is known that IFN-γ binds OprF and triggers cellular responses, increasing the virulence of P. aeruginosa [8]. A permanent inhibitory effect was observed, in response to increasing concentrations of IFN-γ. Furthermore, IFN-γ addition to the OprF proteoliposomes affected OprF secondary structure, producing disordered structures and impairing its activity. This interaction can be a mechanism that allows the bacteria to escape from the host immune system by blocking its OprF pore.
The storage stability of the OprF proteoliposomes was assessed, showing that they could be stored at 4 °C or lower temperatures, for at least two weeks, without protein degradation, therefore meeting the storage conditions necessary of vaccine preservation.
Image of proteoliposome
Efficient immunization with OprF proteoliposomes
Finally, the use of the developed OprF proteoliposome as a potential candidate for a vaccine against P. aeruginosa was evaluated, by immunizing mice with the proteoliposomes and then inoculating them with a lethal dose of the mucoid CF isolate CHA pathogenic strain of P. aeruginosa, with the results showing that the immunization conferred a 90% protection rate. In addition, the OprF proteoliposomes were able to induce the production of neutralizing antibodies, since sera from immunized mice contained polyclonal antibodies capable of recognizing both the recombinant OprF protein in the liposomes and the native protein from bacterial lysates.
The vaccine response of the OprF proteoliposomes against a mucoid strain of P. aeruginosa was higher than the ones reported for other vaccine strategies based on the use of partial OprF protein [17-19], demonstrating the advantages of using the OprF full-length protein, expressed in a cell-free system, without requiring the inclusion of other immunogens. This procedure allowed fostering the occurrence of the rare open-channel conformer, exposing new native epitopes which exist neither in the closed conformer nor in the partial OprF protein. Thus, the OprF proteoliposomes may be a better candidate for a vaccine against P. aeruginosa.
Synthelis
The approach describes above to produce proteoliposomes using a cell-free system was patented worldwide* by the University of Grenoble Alps (UGA) and Synthelis acquired the patent portfolio in 2019. Also, Synthelis benefits from exclusive rights as well as from more than 10 years of development and experience in using this approach. Thus, if we can help you in your project in producing customized proteoliposomes with a specific membrane protein, please contact us in clicking below:
*Patent N°FR0754701, US9,206,456, JP5421241, AUST 2008263765, EU08805711.2
References:
[1] Gaynes R, Edwards JR, National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41 (2005) 848-854. doi:10.1086/432803
[2] Valderrey AD, Pozuelo MJ, Jiménez PA, Maciá MD, Oliver A, Rotger R. Chronic colonization by Pseudomonas aeruginosa of patients with obstructive lung diseases: cystic fibrosis, bronchiectasis, and chronic obstructive pulmonary disease. Diagn. Microbiol. Infect. Dis. 68 (2010) 20-27. doi:10.1016/j.diagmicrobio.2010.04.008
[3] Poole K. Pseudomonas aeruginosa: resistance to the max. Front. Microbiol. 2 (2011) 65. doi:10.3389/fmicb.2011.00065
[4] Pang Z, Raudonis R, Glick BR, Lin T-J, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 37 (2019) 177-192. doi:10.1016/j.biotechadv.2018.11.013
[5] Baker SM, McLachlan JB, Morici LA (2020) Immunological considerations in the development of Pseudomonas aeruginosa vaccines. Hum. Vaccin. Immunother. 16 (2020) 412-418. doi:10.1080/21645515.2019.1650999
[6] Chevalier S, Bouffartigues E, Bodilis J, Maillot O, Lesouhaitier O, Feuilloley MGJ, Orange N, Dufour A, Cornelis P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 41 (2017) 698-722. doi:10.1093/femsre/fux020
[7] Fito-Boncompte L, Chapalain A, Bouffartigues E, Chaker H, Lesouhaitier O, Gicquel G, Bazire A, Madi A, Connil N, Véron W, Taupin L, Toussaint B, Cornelis P, Wei Q, Shioya K, Déziel E, Feuilloley MGJ, Orange N, Dufour A, Chevalier S. Full virulence of Pseudomonas aeruginosa requires OprF. Infect. Immun. 79 (2011) 1176-1186. doi:10.1128/IAI.00850-10
[8] Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, Patel N, Musch MW, Chang EB, Fu Y-X, Jacobs MA, Nishimura MI, Hancock REW, Turner JR, Alverdy JC. Recognition of host immune activation by Pseudomonas aeruginosa. Science 309 (2005) 774-777. doi:10.1126/science.1112422
[9] Brinkman FSL, Bains M, Hancock REW. The amino terminus of Pseudomonas aeruginosa outer membrane protein OprF forms channels in lipid bilayer membranes: correlation with a three-dimensional model. J. Bacteriol. 182 (2000) 5251-5255. doi:10.1128/jb.182.18.5251-5255.2000
[10] Sugawara E, Nagano K, Nikaido H. Alternative folding pathways of the major porin OprF of Pseudomonas aeruginosa. FEBS J. 279 (2012) 910-918. doi:10.1111/j.1742-4658.2012.08481.x
[11] Duchêne M, Schweizer A, Lottspeich F, Krauss G, Marget M, Vogel K, von Specht BU, Domdey H. Sequence and transcriptional start site of the Pseudomonas aeruginosa outer membrane porin protein F gene. J. Bacteriol. 170 (1988) 155-162. doi:10.1128/jb.170.1.155-162.1988
[12] Kimura-Soyema T, Shirouzu M, Yokoyama S. Cell-free membrane protein expression. Methods Mol. Biol. 1118 (2014) 267-273. doi:10.1007/978-1-62703-782-2_18
[13] Zawada JF, Yin G, Steiner AR, Yang J, Naresh A, Roy SM, Gold DS, Heinsohn HG, Murray CJ. Microscale to manufacturing scale-up of cell-free cytokine production: a new approach for shortening protein production development timelines. Biotechnol. Bioeng. 108 (2011) 1570-1578. doi:10.1002/bit.23103
[14] Liguori L, Stidder B, Alcaraz J-P, Lenormand J-L, Cinquin P, Martin DK. Cell-free production of VDAC directly into liposomes for integration with biomimetic membrane systems. Prep. Biochem. Biotechnol. 46 (2016) 546-551. doi:10.1080/10826068.2015.1068800
[15] He W, Felderman M, Evans AC, Geng J, Homan D, Bourguet F, Fischer NO, Li Y, Lam KS, Noy A, Xing L, Cheng RH, Rasley A, Blanchette CD, Kamrud K, Wang N, Gouvis H, Peterson TC, Hubby B, Coleman MA. Cell-free production of a functional oligomeric form of a Chlamydia major outer-membrane protein (MOMP) for vaccine development. J. Biol. Chem. 292 (2017) 15121-15132. doi:10.1074/jbc.M117.784561
[16] Mayeux G, Gayet L, Liguori L, Odier M, Martin DK, Cortès S, Schaack B, Lenormand J-L. Cell-free expression of the outer membrane protein OprF of Pseudomonas aeruginosa for vaccine purposes. Life Sci. Alliance 4 (2021) e202000958. doi:10.26508/lsa.202000958
[17] Hassan R, El-Naggar W, El-Aziz AMA, Shaaban M, Kenawy HI, Ali YM. Immunization with outer membrane proteins (OprF and OprI) and flagellin B protects mice from pulmonary infection with mucoid and nonmucoid Pseudomonas aeruginosa. J. Microbiol. Immunol. Infect. 51 (2018) 312-320. doi:10.1016/j.jmii.2016.08.014
[18] Knapp B, Hundt E, Lenz U, Hungerer K-D, Gabelsberger J, Domdey H, Mansouri E, Li Y, von Specht B-U. A recombinant hybrid outer membrane protein for vaccination against Pseudomonas aeruginosa. Vaccine 17 (1999) 1663-1666. doi:10.1016/S0264-410X(98)00420-4
[19] von Specht BU, Knapp B, Muth G, Bröker M, Hungerer KD, Diehl KD, Massarrat K, Seemann A, Domdey H. Protection of immunocompromised mice against lethal infection with Pseudomonas aeruginosa by active or passive immunization with recombinant P. aeruginosa outer membrane protein F and outer membrane protein I fusion proteins. Infect. Immun. 63 (1995) 1855-1862. doi:10.1128/IAI.63.5.1855-1862.1995
Topics: OprF proteoliposomes, Pseudomonas aeruginosa, vaccine





