.jpg?width=1400&name=protein%20cell%20free%20(1).jpg)
For more than 15 years, cell-free systems have been considered effective for high protein production!
Indeed, we now know much more about the metabolic networks that exploit these systems and also understand how to avoid non-essential activities in order to supply more energy for protein synthesis and avoid substrate limitations (Kim et al., 1999, 2000, 2001). We have therefore optimized the system, and so, energy that would have been previously wasted is now directed into protein production.
In addition, major advances have been made in the cell extract preparation process in order to reduce time and costs associated with its preparation and to improve its capacity to synthesize proteins at high yield. For example, high-density E. coli fermentation has been developed, allowing the preparation of large volume of cell extracts (Zawada et al., 2005).
Furthermore, shorter and more cost-efficient extraction clarification and activation for the cell extract have been developed (Liu et al., 2005) and further optimized. The ability to work on a long term basis with cell extract has been confirmed after a freezing period of one year, with no noticeable loss of functionality. (Zawada et al., 2011).
Moreover, stable and cost-effective energy generating systems providing ATP-regeneration have been developed in order to reduce the cost of cell-free systems; conventional systems use enzymes, co-factors or expensive phosphate donor molecules for ATP regeneration, which limits the use of cell-free systems at an industrial scale.
Alternative systems, such as those activating the central metabolism, using oxidative phosphorylation with glutamate (Jewett et al., 2008) or hexametaphosphate coupled with maltodextrin (Cashera et al., 2015) allow a significant reduction in costs.
Another element often overlooked is the capacity of cell-free systems to generate high yields. Indeed, reaction volumes from 15 µL to 1 mL are often reported, but process parameters have been developed in stirred tank fermenters to perform the synthesis reaction at a 100-liter scale! Equipment and parameters compatible at an industrial scale were used: the reaction was carried out in standard tanks; pH and dissolved oxygen concentration were regulated, foam was reduced, and bacterial growth was inhibited (Zawada et al., 2011).
As a result, the combination of these technological advances in combination with the use of pilot equipment enables the production of active rhGM-CSF in cell-free systems at yields of 700 mg/L in 10 hours at the 100-liter scale. This means that 70 kg of rhGM-CSF was produced in 10 hours at a GMP grade (Zawada et al., 2011).
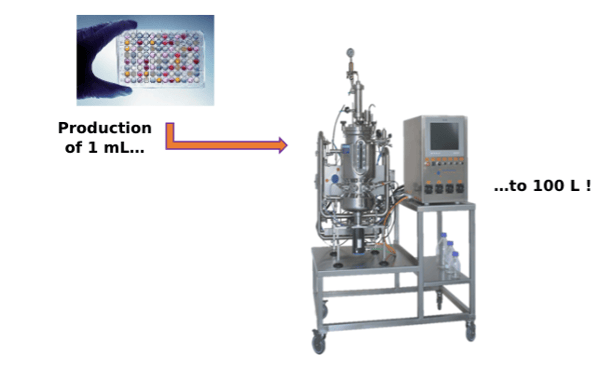
These different results are a clear demonstration that cell-free technology is no longer reserved uniquely to small-scale production, but is now suitable for large scale industrial applications.
Authors & sources :
Carlson E.D., Gan R., Hodgman C.E., Jewett M.C. 2012. Cell-free protein synthesis: Applications come of age. Biotechnology Advances 30:1185–1194.
Cashera F., Noireaux V. 2015. A cost-effective polyphosphate-based metabolism fuels an all E. coli cell-free expression system. Metabolic Engineering 27:29-37.
Jewett M.C, Calhoun K.A., Voloshin A., Wuu J.J., Swartz J.R. 2008. An integrated cell-free metabolic platform for protein production and synthetic biology. Molecular Systems Biology 4(220).
Kim D.M., Swartz J.R. 1999. Prolonging cell-free protein synthesis with a novel ATP regeneration system. Biotechnol Bioeng 66:180–8.
Kim D.M., Swartz J.R. 2000. Prolonging cell-free protein synthesis by selective reagent additions. Biotechnol Prog 16:385–90.
Kim D.M., Swartz J.R. 2001. Regeneration of adenosine triphosphate from glycolytic intermediates for cell-free protein synthesis. Biotechnol Bioeng 74:309–16.
Liu D.V., Zawada J.F., Swartz J.R. 2005. Streamlining Escherichia coli S30 extract preparation for economical cell-free protein synthesis. Biotechnol Prog 21(2):460–465.
Zawada J., Swartz J. 2005. Maintaining rapid growth in moderate-density Escherichia coli fermentations. Biotechnol Bioeng 89(4):407-415.
Zawada J., Swartz J. 2006. Effects of growth rate on cell extract performance in cell-free protein synthesis. Biotechnol Bioeng 94(4):618–624.
Zawada J.F, Yin G., Steiner A.R., Yang J., Naresh A., Roy S.M., Gold D.S., Heinsohn H.G., Murray C.J. 2011. Microscale to manufacturing scale-up of cell-free cytokine production.




